IBBR Researchers Receive $1.1M from FDA and NIIMBL to Support Biomanufacturing Innovation
IBBR Researchers Receive $1.1M from FDA and NIIMBL to Support Biomanufacturing Innovation
Researchers at the Institute for Bioscience and Biotechnology Research (IBBR) recently received two awards totaling $1.1M from the Food and Drug Administration (FDA) and the National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL) to advance the development of analytical methods for characterizing complex drugs and vaccines. IBBR Fellow Dr. Bruce Yu (Professor, Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy) is principal investigator on the awards. His team at IBBR includes Dr. Marc Taraban and Dr. Katharine Briggs.
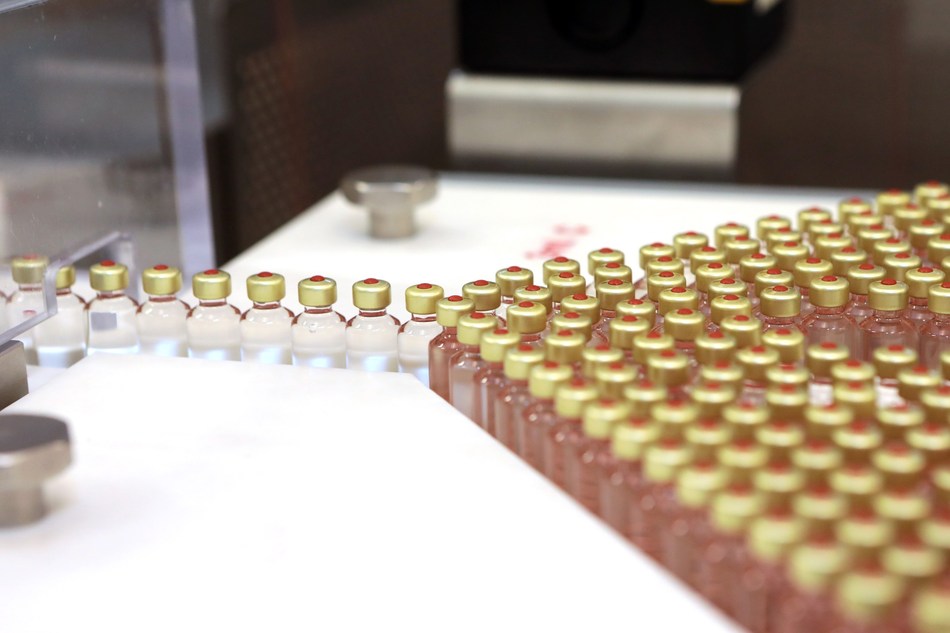
Vaccines, antibodies, and other top-selling therapeutics are biologics—complex biomolecules such as proteins and nucleic acids. Biomanufacturing these products involves lengthy and costly production, purification, and packaging processes. Not only are the final products complex, but the entire biomanufacturing process is complicated and sensitive, requiring the use of advanced instrumentation capable of sophisticated measurements to monitor the process and validate the final product.
Current measurements require contact with the biomanufacturing solution, which introduces additional sampling steps and a risk of contamination. Current quality control practices require opening a subset of vials from each batch and making conclusions about entire batches based on those measurements.
Yu's lab is pioneering the use of water proton nuclear magnetic resonance (wNMR) spectroscopy for biomanufacturing applications and has several issued and pending patents. First developed in 1946, NMR can be thought of as a laboratory MRI—a non-invasive measurement technique utilizing strong magnets. The Yu group uses benchtop, time-domain NMR instruments to measure characteristics of water that are sensitive to changes of the complex biomolecules dissolved in it. "Our goal is to apply these methods to measure important safety and efficacy factors of vaccines and drugs both during and after biomanufacturing," Yu explained.
For example, the team recently demonstrated sensitive, non-destructive measurements of concentration variability within intact insulin pens, as well as the ability to detect changes to packaged vaccines due to freezing, which can lead to decreased effectiveness. In another advance, Yu's team demonstrated the detection of variations in drug concentration and protein aggregation using wNMR under flow conditions that simulate a biomanufacturing system.
With support from NIIMBL, Yu's group, along with scientists from vaccine teams at Merck & Co. Inc. and Pfizer Inc., will adapt their flow wNMR methods to monitor the production and stability of vaccine-adjuvant complexes during biomanufacturing. The new FDA award will support development of additional wNMR-based methods to detect issues that arise during packaging, storage, and transport, such as variability of filling and freezing.
"Novel methods to improve process analytical technologies for vaccines and biologics are critical to the biopharmaceutical industry. I am pleased that IBBR researchers are working with industry and government agencies to develop innovative ways to address these challenges," said IBBR Director Dr. Thomas Fuerst.
November 20, 2019
Prev Next
Connect
Did You Know

UMD is the only major public research university inside the Washington, DC beltway!!
